CATEGORYPlatingGalvanized
Last time, I talked about the variety of metals in electroplating.
This time, I will introduce "galvanization".
Have you ever heard of zinc?
There may be more people who have heard about nutrition rather than metal.
It is said to be included in oystersand natto.

Such a metal called zinc is used as plating.
Galvanization compensates for the weakness of steel, which is "rust-prone".
Not only that, but it is also an excellent thing that leaves the ease of processing that steel originally has.
The cost is relatively low and is used in various places.
It is used for car bodies and parts of cars and motorcycles, tin roofs.
It means that it is strong outdoors.


So how can zinc protect iron from rust?
I expressed galvanization as shown in the illustration below.
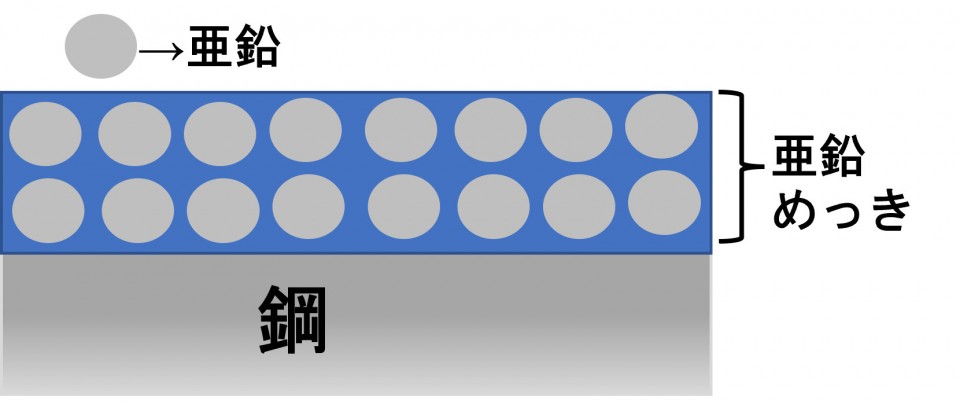
For example, let's say this galvanization is scratched.
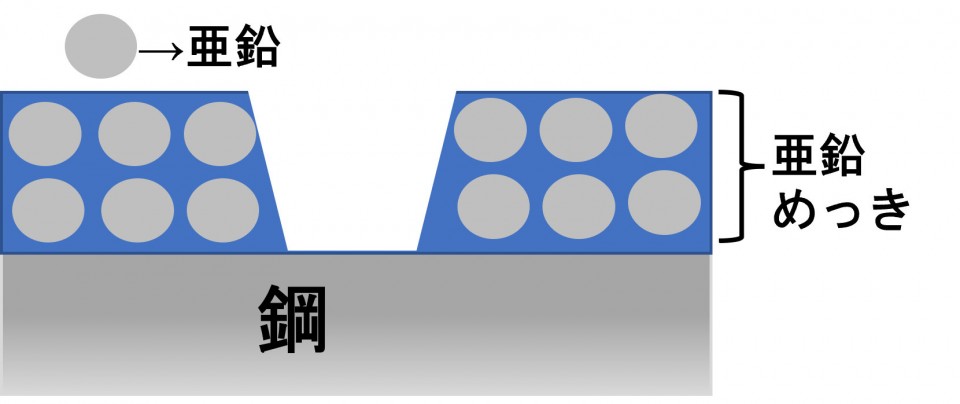
Even if that happens, zinc acts to protect iron before steel rusts.
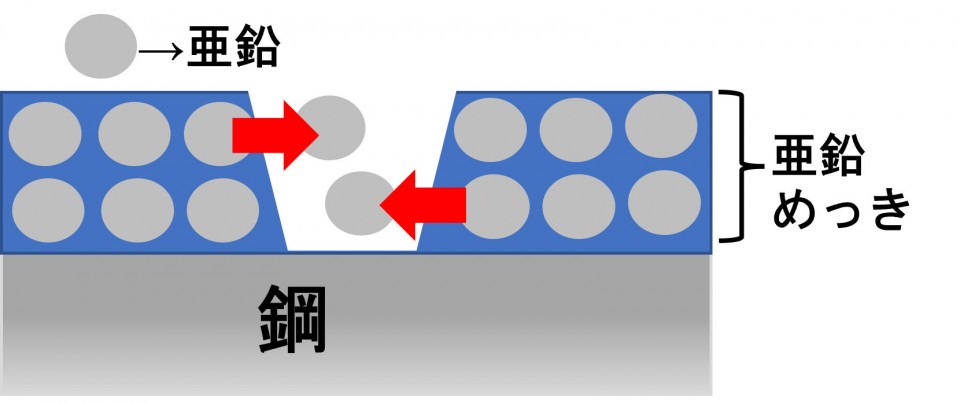
This is called "sacrificial anticorrosion action".
It's cool somehow.




